
| program | Target/Mechanism | Indication | Pre-clinical/ IND Enabling |
Phase I | Phase II | Phase III | Key Regulatory Authorities | Rights |
|---|---|---|---|---|---|---|---|---|
| LP-003 | IgE | Seasonal AR | NMPA | Global | ||||
| CSU | NMPA | |||||||
| Allergic asthma | NMPA | |||||||
| CRSwNP | NMPA | |||||||
| Other allergic diseases | NMPA | |||||||
| LP-00A bi-functional antibody |
undisclosed | Allergic diseases | Global | |||||
| LP-005 | C5xC3b | PNH | NMPA | Global | ||||
| Complement-mediated kidney diseases | NMPA | |||||||
| Other complement related indications | NMPA | |||||||
| LP-00C bi-functional antibody or fusion protein |
undisclosed | B lymphocytes-mediated autoimmune diseases | Global | |||||
| LP-00D bi-functional antibody or fusion protein |
undisclosed | Complement related indications | Global |
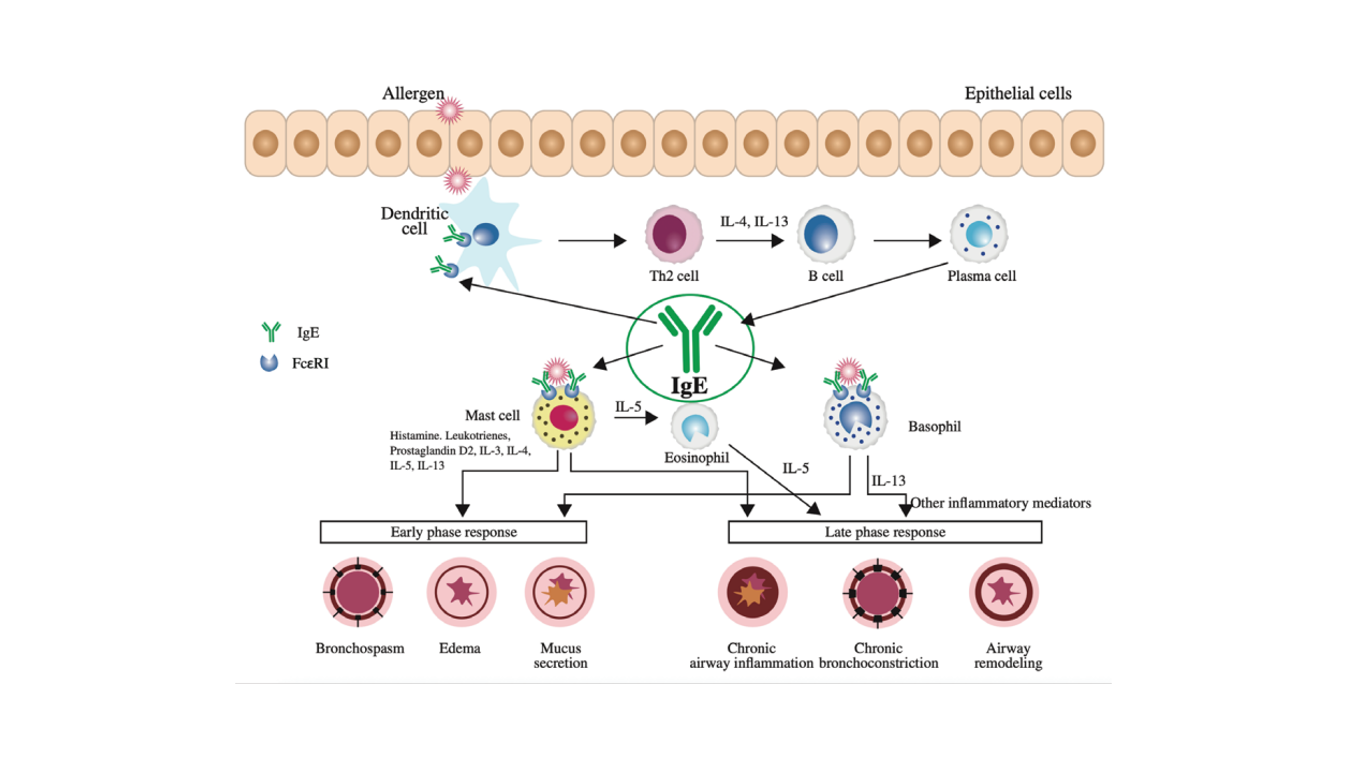
Source: Humbert M , Bousquet J , Bachert C ,et al.IgE-Mediated Multimorbidities in Allergic Asthma and the Potential for Omalizumab Therapy[J].The Journal of Allergy and Clinical Immunology In Practice, 2019, 7(5).DOI:10.1016/j.jaip.2019.02.030.

Source: Gould H J , Bryan Y C .IgE repertoire and immunological memory: Compartmental regulation and antibody function[J].International Immunology, 2018(9):9.DOI:10.1093/intimm/dxy048.
| Items | LP-003 | Omalizumab | Ligelizumab |
|---|---|---|---|
| KD* Affinity to IgE | 2.08 pM | 1790 pM | 12.1 pM |
| FcεRI bioassay | ++++ | ++ | +++ |
| T 1/2 (healthy volunteers) | Q45~75 days | 17~20 days | ~20 days |
| program | Indication | Discovery | Pre-clinical | Phase I | Phase II | Phase III | BLA |
|---|---|---|---|---|---|---|---|
| LP-003 | Seasonal Allergic Rhinitis (SAR) |
|
|||||
| Chronic Spontaneity Urticaria (CSU) |
|
||||||
| Allergic Asthma |
|
||||||
| Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) |
|
||||||
| Other allergic diseases |
|

Source: Posch W , Bermejo-Jambrina M , Lass-Flrl C ,et al.Role of Complement Receptors (CRs) on DCs in Anti-HIV-1 Immunity[J].Frontiers in Immunology, 2020, 11.DOI:10.3389/fimmu.2020.572114..

Source: https://www.theisn.org/initiatives/toolkits/complement-mediated-kidney-disease-toolkit/#Overview-Complement
| program | Indication | Discovery | Pre-clinical | Phase I | Phase II | Phase III |
|---|---|---|---|---|---|---|
| LP-005 | Paroxysmal nocturnal hemoglobinuria (PNH) |
|
||||
| Complement-mediated kidney diseases |
|
|||||
| Other complement related diseases |
|